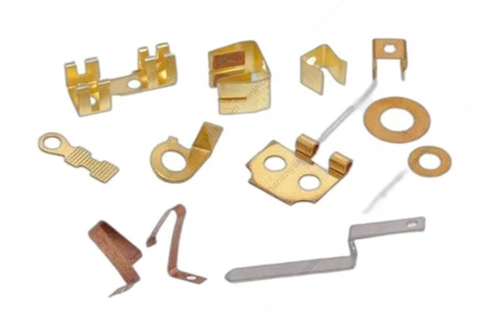
In the field of medical device manufacturing, medical metal stampings are among the core components that must meet extremely high standards in precision, tolerance control, and biocompatibility. As an industry-leading provider of metal stamping services, JCL leverages its strong technical foundation and advanced production systems to deliver high-quality, customizable stamping solutions to clients across the global medical sector.
Core Advantages of JCL Medical Metal Stampings
High-Precision Stamping for Complex Structures
JCL's metal stamping processes tailored for the medical industry offer exceptional precision control. Whether it's intricate implant components or finely structured diagnostic device parts, JCL ensures stable and consistent batch processing to meet the forming requirements of complex and high-difficulty products.
Material Expertise to Ensure Performance Compatibility
JCL has a seasoned engineering team with deep expertise in medical-grade materials, including stainless steel, titanium alloys, aluminum alloys, and more. The team selects the most appropriate materials based on the device's specific functions and environmental demands, ensuring compatibility with medical applications in terms of corrosion resistance, biocompatibility, and mechanical performance.
Customized Solutions for Individual Needs
Medical device development is highly personalized and innovative. JCL supports end-to-end custom services—from prototyping to mass production. Every stamped component is manufactured to match client-provided drawings or specific design requirements. Whether it's unique dimensions, special surface treatments, or specific assembly structures, JCL delivers with precision.
Tight Tolerance Control and Regulatory Compliance
In the medical industry, the precision of part fitment directly affects device safety and functionality. JCL offers tight tolerance stamping to ensure flawless component assembly. All manufacturing processes comply with relevant medical quality management systems, such as ISO 13485 and FDA standards, ensuring that products meet global regulations and market entry requirements.
Biocompatibility Assurance for Patient Safety
JCL understands that medical devices must make direct or indirect contact with the human body. Therefore, materials used in production, as well as coatings and surface treatments, are strictly controlled for biocompatibility and safety. Every step of the process undergoes rigorous testing to ensure no adverse effects on patients.
Typical Applications of Medical Metal Stampings
Surgical instrument components
Metal housings for implantable devices (e.g., pacemaker enclosures)
Frames and brackets for medical monitoring equipment
Accessories for dental and orthopedic implants
Metal components in disposable medical instruments
As the medical device industry continues to push for higher precision, miniaturization, and increased reliability, JCL stands out with its powerful customization capabilities, rigorous quality control, and deep understanding of biosafety. If you're seeking high-performance stamped parts that meet medical standards and can be tailored to your needs, JCL is undoubtedly your ideal partner.